Life's Ingredients Could Form on Titan's Surface
Breaking space news, the latest updates on rocket launches, skywatching events and more!
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered daily
Daily Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!

Twice a month
Strange New Words
Space.com's Sci-Fi Reader's Club. Read a sci-fi short story every month and join a virtual community of fellow science fiction fans!
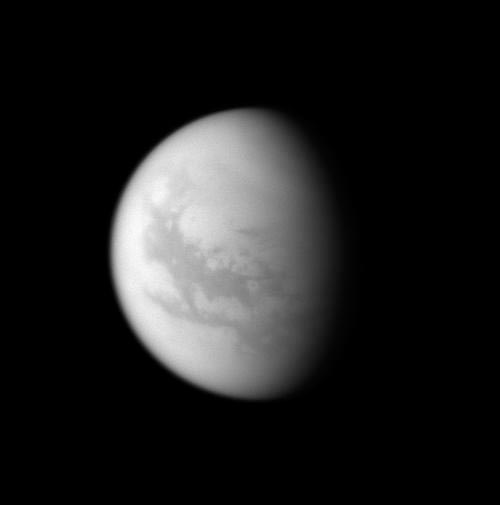
SAN FRANCISCO ? Complex molecules similar to the buildingblocks of life on Earth may be able to form on Saturn's moon Titan, a new studysuggests.
Organic molecules falling out of Titan's atmosphere couldreact with liquid water on the surface of the moon to form biomolecules likeamino acids, according to the study. This liquid water could be delivered bycomet strikes or ice-volcanoeruptions, and it may stay liquid long enough on the frigid moon for suchreactions to take place.
"It's very possible that you could get biomolecules,"said study author Catherine Neish of Johns Hopkins University's Applied PhysicsLaboratory. Neish presented her results here today (Dec. 14) at the 2010 fallmeeting of the American Geophysical Union.
Organics in Titan's air
Lotsof organic molecules swirl about in Titan's nitrogen-rich atmosphere. Thatatmosphere doesn't contain much oxygen, though, so the chances of formingbiomolecules like amino acids in the air aren't great, Neish said.
But those organics fall to Titan's ground all the time.
"They just continually trickle down out of theair," Neish told SPACE.com.
Breaking space news, the latest updates on rocket launches, skywatching events and more!
On the ground, organics might have a chance of mixing withliquid water, which could supply the required oxygen. Wateris not a common feature on Titan, which has liquid-hydrocarbon lakes. Butit could be delivered by events such as comet strikes or ice-volcano eruptions,Neish said.
Titan's surface is a frosty minus 290 degrees Fahrenheit(minus 179 degrees Celsius), so any such water would eventually freeze. Butprevious work by other researchers suggests that water supplied by suchdramatic events might last surprisingly long ? from 100 to 1,000 years ?because there'd be a lot of it.
Neish was interested in knowing whether complex biomoleculesmight be able to form in this timeframe. So in the lab, she mixed Titan-likeorganic molecules with liquid water at low temperatures ? 32 degrees F (0degrees C) for pure water, and minus 4 F (minus 20 C) for an ammonia-water mix.
In both cases, oxygen-containing molecules were producedvery quickly, on the order of several days. Further experiments found that thereactions produced at least four identifiable amino acids: asparagine, asparticacid, glutamine and glutamic acid.
Titan life still a long shot
These hints of complex chemistry are intriguing, but theydon't suggest that the Titansurface is crawling with life, Neish said.
"I'm fairly pessimistic about the prospects of life onTitan," she said. "To sustain life, you need some sort of energysource. And there's just not a lot of energy there."
But she thinks the new study has the potential to yieldinsights about how life could have arisen on Earth, which may have resembledTitan in many ways long, long ago.
The study suggests, for example, that biomolecules arefairly easy to make, even at low temperatures. So it's not too much of astretch to think that life may have taken root in coldenvironments on Earth. An icy surface could protect any nascentbiomolecules from damaging ultraviolet radiation, for example, and possiblyfrom impact events, Neish said.
"Darwin talked about life possibly forming in a warmpond," she said. "Well, maybe a freezing pond is a good place forlife to begin."
- The Strangest Places Where Life Is Found on Earth
- Arsenic-Eating Bacteria Opens New Possibilities for Alien Life
- Images - European Probe Lands on Titan

Michael Wall is a Senior Space Writer with Space.com and joined the team in 2010. He primarily covers exoplanets, spaceflight and military space, but has been known to dabble in the space art beat. His book about the search for alien life, "Out There," was published on Nov. 13, 2018. Before becoming a science writer, Michael worked as a herpetologist and wildlife biologist. He has a Ph.D. in evolutionary biology from the University of Sydney, Australia, a bachelor's degree from the University of Arizona, and a graduate certificate in science writing from the University of California, Santa Cruz. To find out what his latest project is, you can follow Michael on Twitter.
