Neutrons: Facts about the influential subatomic particles
Neutral particles lurking in atomic nuclei, neutrons are responsible for nuclear reactions and for creating precious elements.
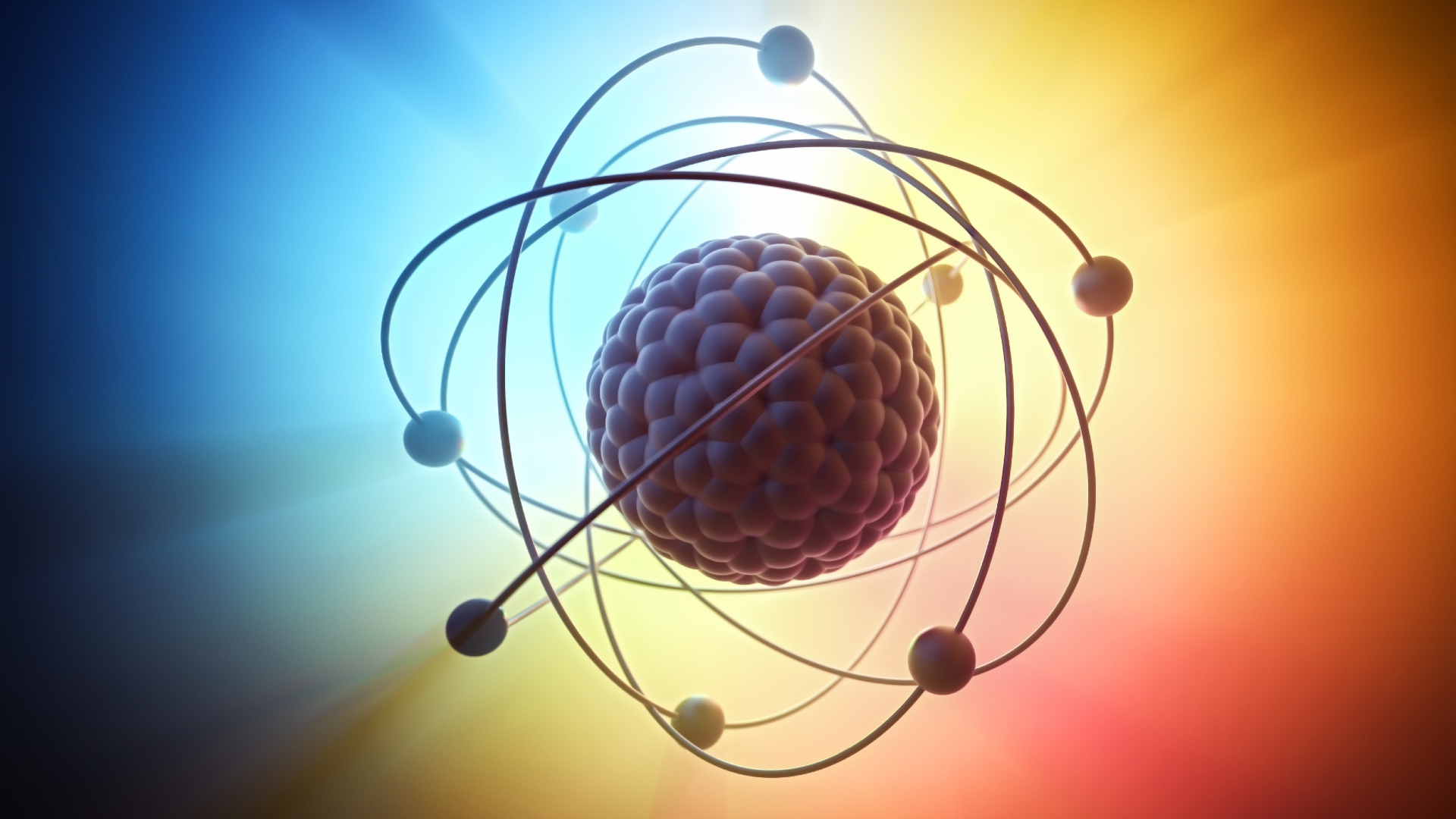
Neutrons are tiny subatomic particles that — along with protons — form the nucleus of an atom.
While the number of protons defines what element an atom is, the number of neutrons in the nucleus can vary, resulting in different isotopes of an element. For example, ordinary hydrogen contains one proton and no neutrons, but the isotopes of hydrogen, deuterium and tritium, have one and two neutrons, respectively, alongside the proton.
Neutrons are composite particles made up of three smaller, elementary particles called quarks, held together by the Strong Force. Specifically, a neutron contains one 'up' and two 'down' quarks. Particles made from three quarks are called baryons, and hence baryons contribute to all the baryonic 'visible' matter in the universe.
Related: What is the theory of everything?
Who discovered neutrons?
After Ernest Rutherford (with help from Ernest Marsden and Hans Geiger's gold-leaf experiment) had discovered in 1911 that atoms have a nucleus, and then nine years later discovered that atomic nuclei are made, at least in part, by protons, the discovery of the neutron in 1932 by James Chadwick naturally followed.
The idea that there must be something else in an atom's nucleus came from the fact that the number of protons didn't match an atom's atomic weight. For example, an oxygen atom contains 8 protons, but has an atomic weight of 16, suggesting that it contains 8 other particles. However, these mystery particles would have to be electrically neutral, since atoms normally have no overall electric charge (the negative charge of the electrons cancels out the positive charge of the protons).
At the time, various scientists were experimenting with alpha particles, which are another name for helium nuclei, bombarding a material made from the element beryllium with an alpha particle stream. When the alpha particles impacted beryllium atoms, they produced mysterious particles that appeared to originate from within the beryllium atoms. Chadwick took these experiments one step further and saw that when the mystery particles hit a target made of paraffin wax, they would knock loose protons at high energy. In order to do this, Chadwick reasoned, the mystery particles must have more or less the same mass as a proton. Chadwick proclaimed this mystery particle to be the neutron, and in 1935 he won a Nobel Prize for his discovery.
Breaking space news, the latest updates on rocket launches, skywatching events and more!
Neutrons: Mass and charge
As their name suggests, neutrons are electrically neutral, so they have no charge. Their mass is 1.008 times the mass of the proton — in other words, it's approximately 0.1% heavier.
Neutrons don't like to exist on their own outside the nucleus. The binding energy of the Strong Force between them and protons in the nucleus keeps them stable, but when out on their own they undergo beta decay after about 15 minutes, transforming into a proton, an electron and an antineutrino.
Albert Einstein, in his famous equation E = mc2, said that mass and energy are equivalent. Although the mass of a neutron and a proton are only slightly different, this slight difference means that a neutron has more mass, and therefore more energy, than a proton and an electron combined. That's why, when a neutron decays, it produces a proton and an electron.
Isotopes and radioactivity
An isotope is a variation of an element that has more neutrons. For instance, at the top of this article, we gave the example of the hydrogen isotopes deuterium and tritium, which have 1 and 2 extra neutrons, respectively. Some isotopes are stable, deuterium for instance. Others are unstable and inevitably undergo radioactive decay. Tritium is unstable — it has a half-life of about 12 years (a half-life is the time it takes on average for half of a given amount of an isotope like tritium to decay), but other isotopes decay far more rapidly, in a matter of minutes, second or even fractions of a second.
Neutrons are also essential tools in nuclear reactions, in particular when inducing a chain reaction. Neutrons absorbed by atomic nuclei create unstable isotopes that then undergo nuclear fission (splitting into two smaller daughter nuclei of other elements). For example, when uranium-235 absorbs an extra neutron, it becomes unstable and breaks apart, releasing energy in the process.
Neutrons are also instrumental in the creation of heavy elements in massive stars, through a mechanism known as the r-process, with "r" meaning "rapid". This process was first detailed in the famous, Nobel Prize-winning B2FH paper by Margaret and Geoffrey Burbidge, William Fowler and Fred Hoyle that described the origins of the elements through stellar nucleosynthesis — the forging of elements by stars.
Stars like the sun can produce elements of oxygen, nitrogen and carbon through nuclear fusion reactions. More massive stars can keep going and create shells of increasingly heavier elements all the way down to iron-56 in the star's core. At this point, the reactions require more energy to be put into them to fuse elements heavier than iron than what is actually produced by those reactions, so those reactions cease, energy production grinds to a halt and the core of the star collapses, instigating a supernova. And it's in the incredibly violent blast of a supernova that conditions can become extreme enough to liberate lots of free neutrons in a short space of time.
In the supernova blast, atomic nuclei are then able to sweep up all these free neutrons before they all decay (this is why it's described as rapid), to instigate r-process nucleosynthesis. Once the nuclei are full of neutrons they turn unstable and undergo beta decay, transforming those extra neutrons into protons. The addition of these protons changes the type of element that a nucleus is, hence it's a way of creating new, heavy elements such as gold, platinum and other precious metals. The gold in your jewelry was made billions of years ago by rapid neutron capture in a supernova!
Neutron stars
As we have seen, only in the most extreme conditions can neutrons survive outside of atomic nuclei, and there are very few places in the universe more extreme than neutron stars. As their name suggests, these are objects made almost entirely of neutrons.
Neutron stars are what is left of the core of a star after it has undergone core collapse and exploded as a supernova. The explosion may have carried away the outer layers of the star, but the contracting core remains intact.
With no nuclear reactions to generate energy to counteract gravity, the mass of the core is so great that it undergoes a catastrophic gravitational collapse in which the gravitational pressure is great enough that protons and electrons are able to overcome the electrostatic force between them and smush together, merging to form neutrons in a kind of reverse beta decay. Almost all the atoms in the core turn into neutrons, hence why we call the result a neutron star. They are small, just 6-12 miles (10-20 km) across, yet they pack in the entire mass of the dead star's core.
The most massive neutron star yet found has a mass 2.35 times greater than our sun, all crammed into a tiny volume. If you could scoop a spoonful's worth of material from the surface of a neutron star, that spoonful would weigh as much as a mountain on Earth!
Binary neutron star mergers, which are detectable as kilonovae and via their gravitational waves, are also sites of copious r-process nucleosynthesis. The kilonova of two merging binary stars that released the gravitational-wave burst GW 170817 produced 16,000 times the mass of Earth in the form of r-process heavy elements, including ten Earth masses' worth of gold and platinum, which is extraordinary!
Follow Keith Cooper on Twitter @21stCenturySETI. Follow us on Twitter @Spacedotcom and on Facebook.
Additional resources
Learn more about neutrons with the U.S. Department of Energy. Explore how neutrons are used in experiments that study condensed matter with the UK Science Technology Facilities Council. Read the famous B2FH paper about the creation of elements inside stars with the help of neutron capture.
Bibliography
Particle Physics, by Brian R. Martin (2011, One-World Publications)
The Cambridge Encyclopedia of Stars, by James R. Kaler (2006, Cambridge University Press):
Collins Internet-Linked Dictionary of Physics (2007, Collins)
This month in physics history. American Physical Society Sites, APS News, Volume 16, number 5. Accessed Dec. 1, 2022, from https://www.aps.org/publications/apsnews/200705/physicshistory.cfm
Neutron decay. Science Direct. Accessed Dec. 1, 2022, from https://www.sciencedirect.com/topics/physics-and-astronomy/neutron-decay

Keith Cooper is a freelance science journalist and editor in the United Kingdom, and has a degree in physics and astrophysics from the University of Manchester. He's the author of "The Contact Paradox: Challenging Our Assumptions in the Search for Extraterrestrial Intelligence" (Bloomsbury Sigma, 2020) and has written articles on astronomy, space, physics and astrobiology for a multitude of magazines and websites.