The first law of thermodynamics: What is it?
The amount of energy in the universe is constant and can neither be destroyed nor created, that's what the first law of thermodynamics tells us.

The first law of thermodynamics tells us the amount of energy in the universe is constant and can neither be destroyed nor created.
The evolution of the universe is therefore about a constant transformation of energy from one form to another. But no matter how many stars and planets the universe creates, how many civilizations spring up into existence on these planets, there will always be the same amount of energy as there was a second after the Big Bang.
Energy transformation is what makes our world work. Humans and animals transform the chemical energy of the food they eat into the kinetic energy of their motion and action and the energy of the chemical processes in their cells. Green plants absorb energy from the sun and turn it into chemical energy in the form of oxygen and sugars that build up their tissues — in a process known as photosynthesis. Solar power plants use the same sunlight to produce electricity. The sun that keeps us all alive burns hydrogen in its core to produce the light and heat we need, dissipating its energy slowly and gradually into the surrounding space.
Related: Phantom energy and dark gravity: Explaining the dark side of the universe
History of the first law of thermodynamics
The first law of thermodynamics, however, did not emerge from the study of the universe, but from efforts of 17th and 18th-century scientists to understand the nature of heat, according to physicist Stephen Wolfram. Various ideas were floated, including that heat might be a fluid-like substance or a result of microscopic particles that make up the matter that we see. By the early 19th century, scientists settled on the understanding that heat is a form of energy.
At that time, the steam engine was a hot new technology, relying on heat to transform water into steam that could then set into motion complex mechanical contraptions capable of performing all sorts of tasks, from propelling locomotives to powering factory equipment. Many gifted brains of the era, therefore, busied themselves with the question of how to make this heat-reliant technology more efficient.
The first person to lay a foundation to what would later become the first law of thermodynamics was German physicist Rudolf Clausius, according to St. Andrews University. In 1850, Clausius published a paper that would make him famous. The name of the paper is a bit of a mouthful: "On the Moving Force of Heat and the Laws of Heat which may be Deduced Therefrom."
Breaking space news, the latest updates on rocket launches, skywatching events and more!
In this paper, Clausius stated that "in all cases in which work is produced by the agency of heat, a quantity of heat is consumed which is proportional to the work done; and conversely, by the expenditure of an equal quantity of work an equal quantity of heat is produced."
The steam engine and the first law of thermodynamics
But what does Clausius’ statement mean exactly? Let's have a look at the good old steam engine.
A steam engine consists of a chamber with a movable piston. The chamber may contain water or some gas. When the chamber is heated up using an external source of heat, the gas inside expands (the water turns into steam), the increasing heat causes more expansion of the gas, which then causes the piston to move. The piston on the outside of the engine then produces useful work (such as setting a locomotive's wheels in motion).
In reverse, by applying an external force on the piston, one can compress the gas inside, which would cause it to heat up. In both cases, the amount of heat either used or generated would be equal to the amount of work applied or delivered. The total energy of the engine and its surroundings will remain constant.
The first law of thermodynamics can be captured by the following equation: ΔU = Q — W, where ΔU is the change in the internal energy, Q is the heat added to the system, and W is the work done by the system.
The total energy of the system is equal to the heat supplied minus the amount of work performed. Work and heat are the processes that add or subtract energy.
Thermodynamics and the role of heat
The discipline that sprung up from the works of Clausius and other physicists of this era, including Britain's William Thomson (later known as the first Baron Kelvin) and France's Sadi Carnot, became known as thermodynamics. Heat plays a central role in thermodynamics, acting as the force that transforms energy from its raw form (think about coal, for example) into actual mechanical work (the movement of a locomotive).
Thermodynamics studies not just the relationship between heat and mechanical work, but also the role of temperature, volume and pressure in the energy exchange.
A thermodynamic system has its enthalpy, which is the sum of its internal energy combined with the effects of its pressure and volume, according to NASA.
Entropy is the measure of the system's ability to perform work, based on its orderliness. Essentially, systems differ in the amount of work they can perform per unit of thermal energy depending on how organized they are.
The so-called Helmholtz free energy of a thermodynamic system describes how much "useful" work a closed thermodynamic system can produce at a constant temperature and volume.
The Gibbs free energy, on the other hand, describes the maximum reversible work that may be performed by a thermodynamic system at a constant temperature and pressure.
These four qualities plus energy are used to describe properties of all thermodynamic systems.
Thermodynamic systems
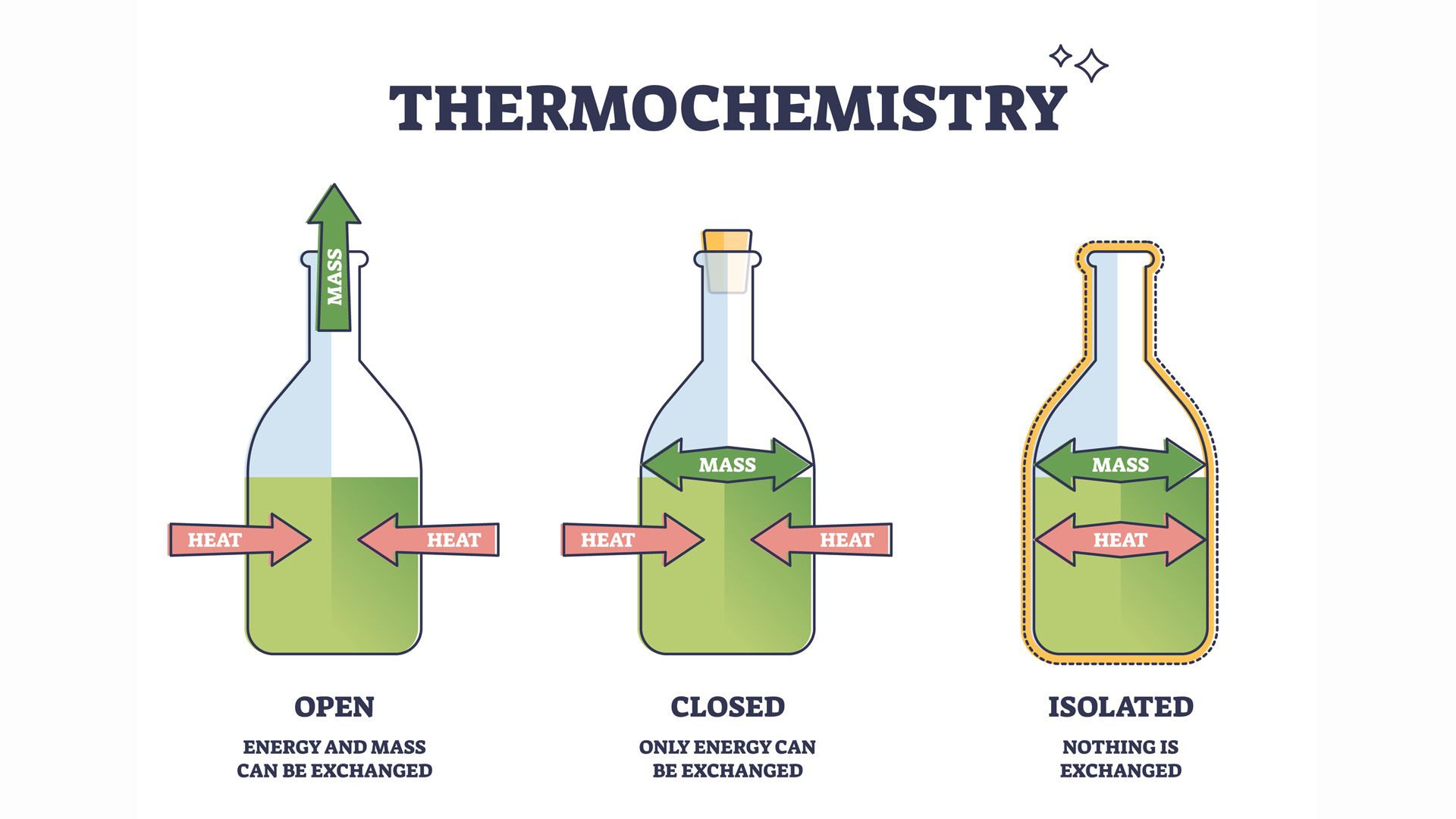
There are three types of systems in thermodynamics: closed, isolated and open.
Isolated systems essentially do not exist. The only truly isolated system being the universe itself.
Closed systems intend to be as much isolated from their surroundings as possible and only exchange energy, but not matter, with their surroundings. A steam engine would be a closed system, but so would be a cooling thermal flask with tea or a white dwarf star gradually losing heat to the vacuum of space.
All living organisms are open systems, exchanging both energy (heat) and matter (food, perspiration, air) with their environment.
Additional resources
Read more about the first law of thermodynamics on our sister website Live Science. Or watch this fun video by the Royal Institution. Explore all three laws of thermodynamics with the educational website Lumen Learning.
Bibliography
Smith, C.W, William Thomson and the Creation of Thermodynamics: 1840-1855, History of Exact Sciences, Springer, 1977
https://www.jstor.org/stable/41133471
Hareesh, T. et al., First law of thermodynamics and emergence of cosmic space in a non-flat universe, Journal of Cosmology and Astroparticle Physics, December 2019 https://iopscience.iop.org/article/10.1088/1475-7516/2019/12/024
Zohuri, B., First Law of Thermodynamics, Physical Chemistry, 2017 https://www.sciencedirect.com/topics/chemistry/first-law-of-thermodynamics
Britannica, The first law of thermodynamics https://www.britannica.com/science/thermodynamics/The-second-law-of-thermodynamics
Follow Tereza Pultarova on Twitter @TerezaPultarova. Follow us on Twitter @Spacedotcom and on Facebook.

Tereza is a London-based science and technology journalist, aspiring fiction writer and amateur gymnast. She worked as a reporter at the Engineering and Technology magazine, freelanced for a range of publications including Live Science, Space.com, Professional Engineering, Via Satellite and Space News and served as a maternity cover science editor at the European Space Agency.
