Did Mercury once have the ingredients for life?
Volatiles may have once cracked Mercury's surface.
Breaking space news, the latest updates on rocket launches, skywatching events and more!
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?
It's possible that Mercury once held the ingredients for life.
In 1974, NASA's Mariner 10 probe flew by Mercury and observed a cracked, cratered landscape. Now, according to one new theory, Mercury's fractured "chaotic terrain" could've been formed by volatiles — elements and compounds that can easily jump from a gas to a liquid or solid — under the surface.
Volatiles, a chemical category that includes water, are essential for sparking and supporting life as we know it here on Earth. So, their potential presence on Mercury is an intriguing development. The study, led by Alexis P. Rodriguez, a researcher at the Planetary Science Institute in Arizona, took a closer look at Mercury'schaotic terrain and the possibility that volatiles once shaped a planet with surface temperatures hot enough to melt lead, a planet that has forever been thought of as "completely inhospitable."
Video: Ice on Mercury – How does it form?
Related: Photos of Mercury from NASA's Messenger spacecraft
What cracked Mercury?
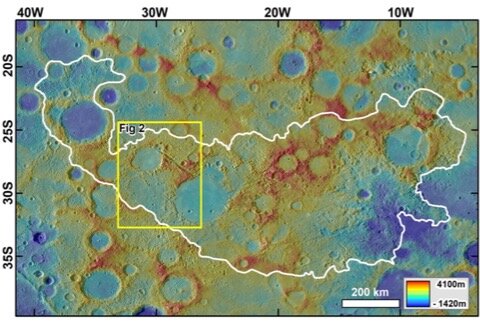
For decades, scientists have studied Mercury's chaotic terrain — a landscape made up of the planet's cracked and ragged surface, complete with jagged, broken-up rocks, sharp peaks and craters.
Mars has chaotic terrain as well, but the Red Planet features connect much more obviously to outflow channels on the Martian surface. With no such channels on Mercury, researchers instead tied the terrain to a powerful asteroid impact that left the Caloris basin, a massive crater still visible on the planet's surface. Until now, it was thought that Mercury's chaotic terrain was created by earthquakes that ravaged Mercury following the impact.
But in the new study, researchers suggest that this couldn't be possible because the timing just doesn't make sense.
Breaking space news, the latest updates on rocket launches, skywatching events and more!
"A key to the discovery was the finding that the development of the chaotic terrains persisted until approximately 1.8 billion years ago, 2 billion years after the Caloris basin formed," co-author Daniel Berman, also of the Planetary Science Institute, said in a statement.
This was the "first, kind of, smoking gun," Rodriguez told Space.com. The researchers were able to date these features using data and images from NASA's MESSENGER (MErcury Surface Space ENvironment GEochemistry and Ranging) spacecraft, which studied Mercury from orbit from 2011 to 2015. The team determined the ages of surface features including the chaotic terrain and the crater formed from the asteroid impact.
Additionally, as Rodriguez explained, the scientists noticed that there were many small features like tiny craters still intact in these terrains. With such a massive asteroid impact, "think about the worst earthquakes you could ever imagine," Rodriguez said. "Instead of bringing down buildings you're bringing down mountains … entire mountain ranges."
The researchers reasoned that, if earthquakes following the impact caused the chaotic terrain, then those smaller features wouldn't have been preserved. These observations informed the team/s findings that this previous notion about the chaotic terrain's formation was flawed.
Missing material
But observations have also shown that some parts of the surface features have dropped, as if something below the surface simply gave way. And, as Rodriguez put it, when earthquakes cause buildings to fall, that matter spreads out over the surface. But in this case, they found that there was a whole bunch of matter that should've been in the chaotic terrain that seemed to be missing.
Keeping the conservation of matter in mind, the team suggests in this study that, instead of an impact and earthquakes causing the planet's surface to crack, volatiles under the surface created these features.
"In this case, we're seeing very clear drops in elevation, very, very abrupt surface losses that indicate that the materials were removed somehow," Rodriguez said.
They concluded that it's much more likely that volatiles underneath Mercury's surface were heated by magma even farther down. Because of this heating, the volatiles became gaseous and sublimated.
"Maybe these materials were volatile and they were transported away, and they could've condensed in other parts of the planet, or maybe they were entirely removed from the planet by solar winds," he added.
The sudden loss of this material is what the scientists think caused the planet's surface to crack and fall apart, creating the chaotic terrain we see today.
So how could volatiles, which are essential to life here on Earth, survive on a planet whose daytime temperatures soar to 800 degrees Fahrenheit (430 degrees Celsius) and drop to minus 290 degrees F (minus 180 C) at night?
While the planet's surface swings wildly between temperature extremes, just below the surface, temperatures are milder, Rodriguez explained.
Rodriguez said that it's "likely that there was some sort of water that formed" in part of Mercury's crust. But the team cannot yet tell exactly what volatiles were present when the planet's surface broke apart.
However, not only are the researchers continuing this work to find an answer to that question, they are also working to understand a much more recent phenomenon.
The future on Mercury
While these events happened billions of years ago, "there is evidence of recent volatile removal within the chaotic terrain … maybe ongoing right now," Rodriguez said.
He explained that, following this study, researchers will be working on "the possibility of selecting a landing site where we could potentially sample these volatile-rich materials using some type of lander."
This work was published March 16 in the journal Scientific Reports.
- How Far is Mercury From the Sun?
- See the Sharpest-Ever View of Mercury's Transit Across the Sun
- Mercury Transit of the Sun: Why Is It So Rare?
Follow Chelsea Gohd on Twitter @chelsea_gohd. Follow us on Twitter @Spacedotcom and on Facebook.
OFFER: Save at least 56% with our latest magazine deal!
All About Space magazine takes you on an awe-inspiring journey through our solar system and beyond, from the amazing technology and spacecraft that enables humanity to venture into orbit, to the complexities of space science.

Chelsea “Foxanne” Gohd joined Space.com in 2018 and is now a Senior Writer, writing about everything from climate change to planetary science and human spaceflight in both articles and on-camera in videos. With a degree in Public Health and biological sciences, Chelsea has written and worked for institutions including the American Museum of Natural History, Scientific American, Discover Magazine Blog, Astronomy Magazine and Live Science. When not writing, editing or filming something space-y, Chelsea "Foxanne" Gohd is writing music and performing as Foxanne, even launching a song to space in 2021 with Inspiration4. You can follow her on Twitter @chelsea_gohd and @foxannemusic.