AI chemist finds molecule to make oxygen on Mars after sifting through millions
The system calculated more than 3.7 million molecules it could make from six different metallic elements in the rocks.

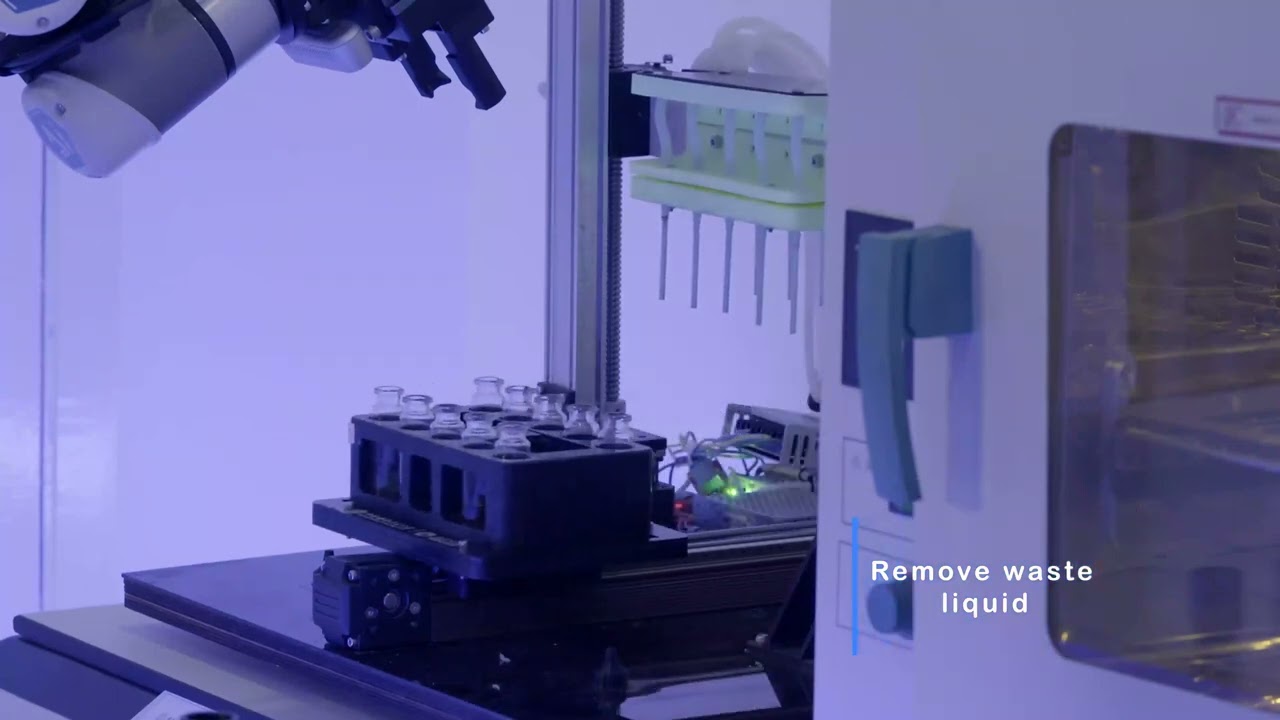
Using meteorites from Mars, an AI-powered robot chemist synthesized compounds that could be used to generate oxygen from water, scientists announced on Monday (Nov. 13).
Potential future crewed missions to Mars will need oxygen — not just for astronauts to breathe, but also for use as rocket propellant. One key way to make such missions cost-effective in the long run is to use resources that already exist on the Red Planet to create the oxygen. That'd be much easier than lugging a bunch of oxygen, and oxygen-producing materials, all the way from Earth.
The idea is promising because Mars does possess significant reserves of frozen water ice — because water is made of hydrogen and oxygen, scientists have been looking for ways to harvest the latter element from those Martian reserves. In particular, compounds known as catalysts are capable of spurring chemical reactions that "split" water molecules to generate oxygen and hydrogen gas.
Related: Mars ice deposits could pave the way for human exploration
In a new study, researchers experimented with an AI chemist to produce some of those water-splitting catalysts — most importantly, these tests were conducted with materials found on Mars. The team focused on five different categories of Martian meteorites, which are rocks that crashed down on Earth after cosmic impacts blasted them off the Red Planet.
The AI chemist used a robot arm to collect samples from the Martian meteorites, then it employed a laser to scan the ore. From there, it calculated more than 3.7 million molecules it could make from six different metallic elements in the rocks — iron, nickel, manganese, magnesium, aluminum and calcium.
Within six weeks, without any human intervention, the AI chemist selected, synthesized and tested 243 of those different molecules. The best catalyst the robot found could split water at minus 34.6 degrees F (minus 37 degrees C), the kind of cold temperature found on none other than Mars.
Breaking space news, the latest updates on rocket launches, skywatching events and more!
"When I was a boy, I dreamed of interstellar exploration," Jun Jiang, co-senior author of the study and a scientist at the University of Science and Technology of China in Hefei. told Space.com. "So when we finally saw that the catalysts made by the robot could actually produce oxygen by splitting water molecules, I felt like my dream was coming true. I even started to imagine that I, myself, will live on Mars in the future."
The researchers estimate it would have taken a human scientist something like 2,000 years to find that "best" catalyst using conventional trial-and-error techniques. Still, Jiang noted that, although these findings suggest AI can be very helpful in science, it "at the same time needs the guidance of human scientists. The robot AI chemist is smart only if we taught it to do something."
The scientists now aim to see if their AI chemist can operate under Martian conditions other than temperature, "in which the atmospheric composition, air density, humidity, gravity and so on are so different than those on Earth," Jiang said.
The researchers detailed their findings online on Monday (Nov. 13) in the journal Nature Synthesis.

Charles Q. Choi is a contributing writer for Space.com and Live Science. He covers all things human origins and astronomy as well as physics, animals and general science topics. Charles has a Master of Arts degree from the University of Missouri-Columbia, School of Journalism and a Bachelor of Arts degree from the University of South Florida. Charles has visited every continent on Earth, drinking rancid yak butter tea in Lhasa, snorkeling with sea lions in the Galapagos and even climbing an iceberg in Antarctica. Visit him at http://www.sciwriter.us