How rainbow-chasing chemists put the twinkle in modern astronomy's eye
Breaking space news, the latest updates on rocket launches, skywatching events and more!
You are now subscribed
Your newsletter sign-up was successful
Want to add more newsletters?

Delivered daily
Daily Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Once a month
Watch This Space
Sign up to our monthly entertainment newsletter to keep up with all our coverage of the latest sci-fi and space movies, tv shows, games and books.

Once a week
Night Sky This Week
Discover this week's must-see night sky events, moon phases, and stunning astrophotos. Sign up for our skywatching newsletter and explore the universe with us!

Twice a month
Strange New Words
Space.com's Sci-Fi Reader's Club. Read a sci-fi short story every month and join a virtual community of fellow science fiction fans!
Life for the 19th-century astronomer was kind of monotonous, 200 years after Galileo's revolutionary work with the telescope.
Astronomers had carefully tracked the motions of the planets, discovered a couple previously unknown planets and a slew of moons, watched stars die, sketched nebulas galore, and more. But they were befuddled by one very simple question: What are the stars, planets and nebulas made of?
It took a few decades of development to find a technique to answer that question, and surprisingly few astronomers were involved in the process. But once the physicists and chemists who were involved invented spectroscopy, it was off to the astronomical races.
Related: 50 fabulous deep-space nebula photos
Over the rainbow
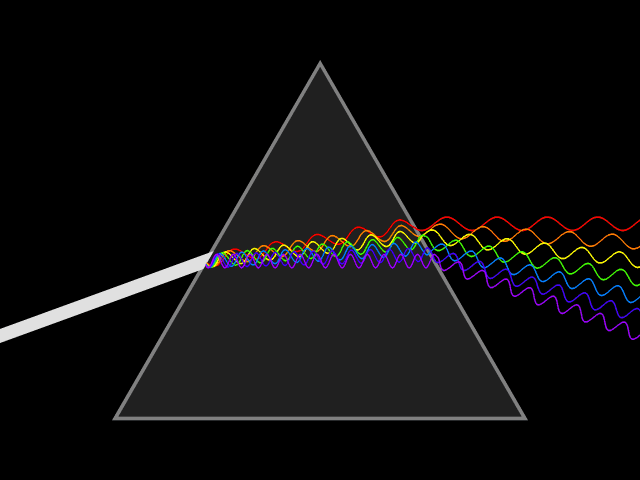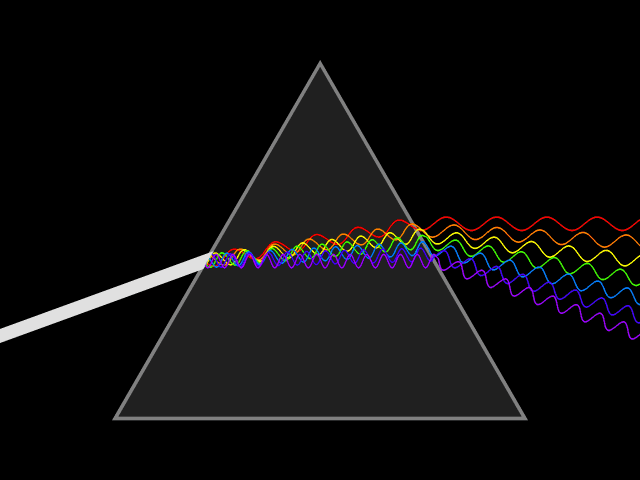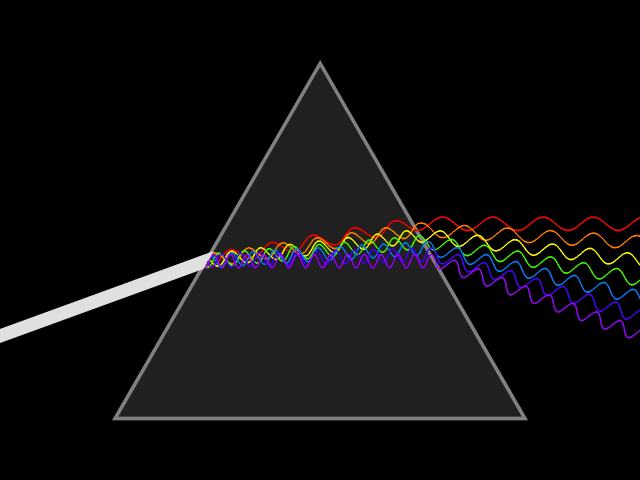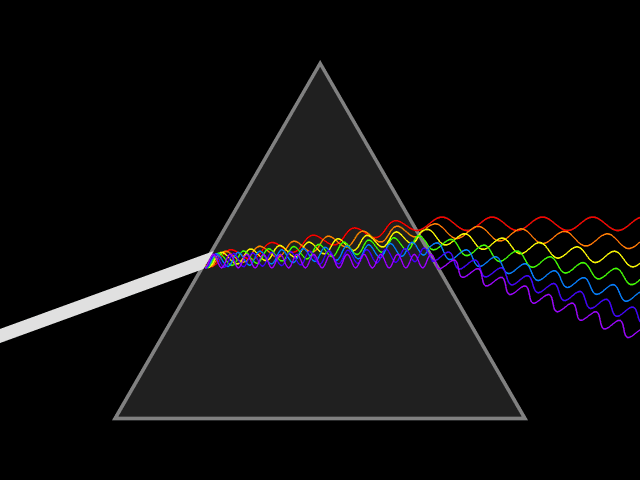
Prisms are fascinating: White light goes in and a rainbow comes out. But for a long time, people assumed that the prism was somehow generating those colors.
It wasn't until Isaac Newton's experiments that we realized that the colors of the rainbow actually come from the white light itself. Newton coined the word "spectrum" to refer to the breakdown of individual components of a given light source: how much blue light it contains, how much red, how much yellow and so on.
But in past centuries, light sources were pretty hard to come by, and early scientists had really only two sources available: the sun and fire. In the mid-1800s, a physicist named Joseph von Fraunhofer decided to play a little game of compare and contrast with those sources.
Breaking space news, the latest updates on rocket launches, skywatching events and more!
Using the best dang prisms he could design (ones that were free from defects and impurities), he compared sunlight to light from a flame, finding some surprising differences. Most notably, he found that the solar spectrum features hundreds of narrow, dark bands scattered across it. Whatever the sun was made of, it was different than a fire.
Fraunhofer went on to perfect an absolutely critical piece of technology known as a diffraction grating, which is a tiny screen with hundreds (or more) of parallel slits carved into it. Light passing through these slits separates into its component colors, producing a spectrum on the other side, exactly like a prism but of much better quality.
Bunsen's burner
A few decades later, a couple of chemists, named Gustav Kirchhoff and Robert Bunsen, began systematically examining the spectra of various elements by dropping the substances into flames (using Bunsen's eponymous burner).
The two scientists found something absolutely beautiful: Each element had its own distinct spectrum, completely distinguishable from the spectrum of any other element. Bunsen and Kirchoff realized they could use spectra to identify the elements and molecules they studied.
This is when the astronomers took to the new method and attached diffraction gratings to the business ends of their telescopes. Point the telescope at a distant object (the sun, a planet, a nebula, a star, the whole deal), and out comes a spectrum, astronomers found. Match up the lines in that spectrum with those produced by a known element, and voila: You can see what stuff in space is made of.
Revealing the universe
Physicists didn't crack the code of how and why spectra are produced for many more decades. But that didn't matter for the astronomers, who say, "Who cares how something works as long as it, you know, works?"
But it turns out that the quantum mechanical nature of an element's subatomic innards causes each unique spectrum. Inside atoms and molecules, energy is quantized. For example, an electron can have only certain specific energy states, not any old energy it wants. The same goes for the vibration or rotation of a molecule; these features can have only certain values.
Sometimes the element can get excited, sucking up energy from some other source, which pops the electrons in the element's atoms up to a higher energy level. Before long, the electrons make their way back down the energy ladder, emitting radiation as they go. Because of the discrete differences between the energy levels, the emitted radiation has very specific wavelengths — bright lines in the spectrum.
But if you place a cloud of a particular element in front of a bright light source, then some of the radiation passing through will strike those atoms and get absorbed. Again, since those jumps can happen only at specific energy levels, the absorbed radiation will be of only very specific wavelengths — dark, missing lines in the spectrum.
Spectroscopy is now an invaluable tool for modern astronomers. It is how we know that the sun contains helium, that the cloud tops of Jupiter contain ammonia, that distant stars are made of the same material as the sun and that nebulas contain the key ingredients for life.
What's more, spectra have revealed a universe in constant motion. The spectra of objects moving away from us pick up a reddish tinge, whereas the spectra of objects moving toward us are tinted blue, phenomena known as redshift and blueshift. Astronomers have used spectra to map the motion of stars inside galaxies and galaxies within the universe. This has led to the realization of phenomena like dark matter and the expansion of the cosmos itself.
All from a bunch of physicists playing around with rainbows.
- The problems with modern physics
- Is it a wave or a particle? It's both, sort of.
- Make your own spectroscope
Learn more by listening to the episode "How Did Spectroscopy Revolutionize Astronomy?" on the "Ask a Spaceman" podcast, available on iTunes and on the web at http://www.askaspaceman.com. Thanks to Shann U. and Stephen for the questions that led to this piece! Ask your own question on Twitter using #AskASpaceman or by following Paul @PaulMattSutter and facebook.com/PaulMattSutter.
Paul M. Sutter is an astrophysicist at SUNY Stony Brook and the Flatiron Institute, host of "Ask a Spaceman" and "Space Radio," and author of "Your Place in the Universe." Follow us on Twitter @Spacedotcom and on Facebook.

Paul M. Sutter is a cosmologist at Johns Hopkins University, host of Ask a Spaceman, and author of How to Die in Space.