Time Crystals Are (Not) Interesting
Paul Sutter is an astrophysicist at The Ohio State University and the chief scientist at COSI Science Center. Sutter is also host of Ask a Spaceman, We Don't Planet and COSI Science Now. Sutter contributed this article to Space.com's Expert Voices: Op-Ed & Insights.
Normally, I wouldn't use these articles to talk about topics in the field of condensed-matter physics — as fascinating as that branch of science can be — but time crystals are just too good to resist. Besides the ridiculous name, which sounds like something out of a bad "Lord of the Rings" knockoff, the physics behind time crystals reveals some important facets of the way the universe at large works.
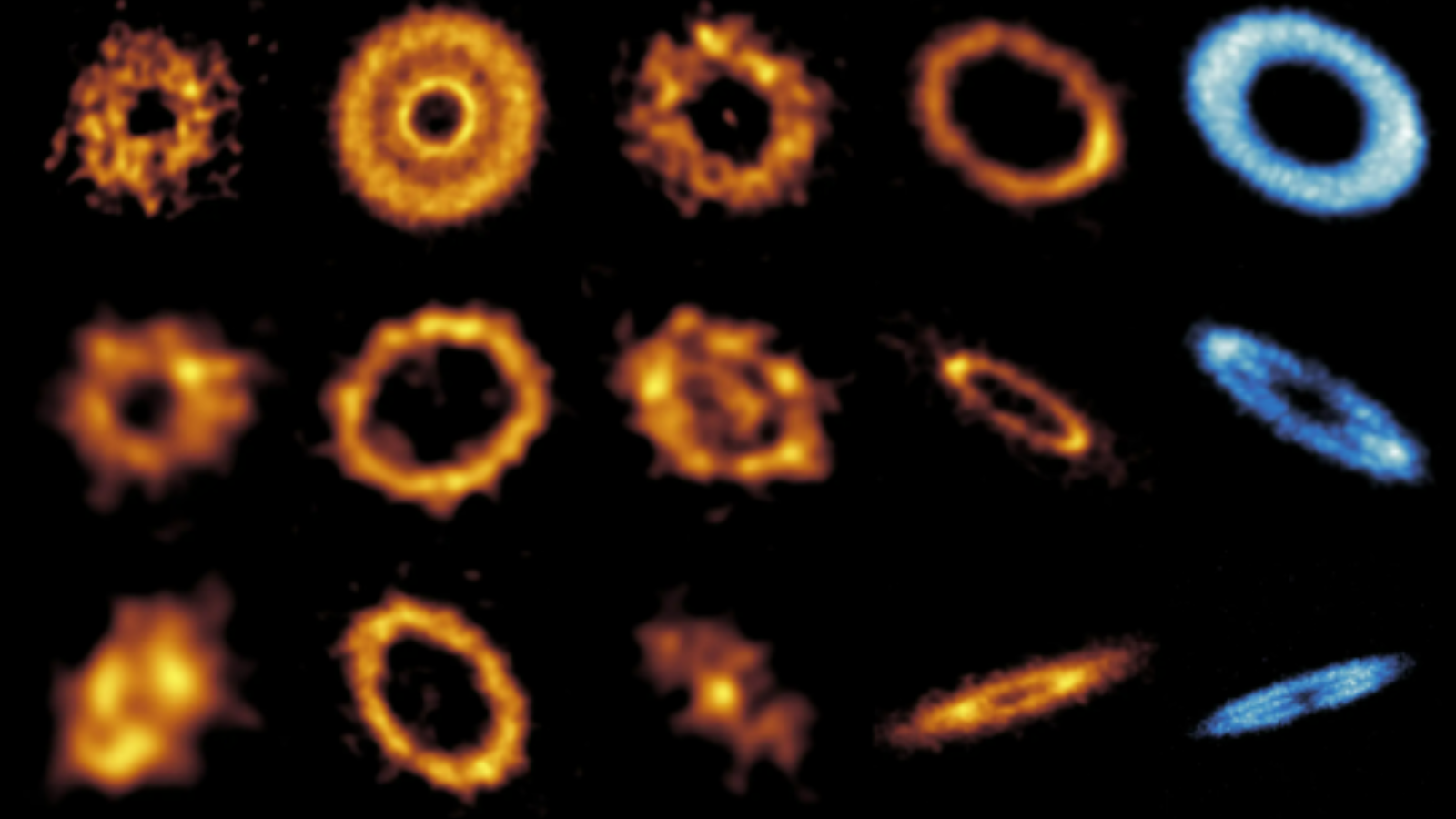
But first, a simple definition. Regular, normal crystals (which are still going to be called just "crystals" instead of "space crystals," I guess) are an arrangement of atoms or molecules that form a regular, repeated pattern in space. A time crystal is, as you might expect, an arrangement of atoms or molecules that form a regular, repeated pattern in time. They'll sit in one configuration for a while, then flip to another, back and forth and back and forth. [In Photos: Bizarre New Time Crystals Created]
While we have a lot of examples of repeated patterns in nature, time crystals are different beasts for two important reasons. For one, they are a stable pattern — the system is in its lowest possible energy state, which means that in principle, they can keep oscillating forever.
This sounds suspiciously like a perpetual-motion machine — hence all the buzz.
That, and the fact that they violate the law of conservation of energy.
A broken symmetry
The pioneering work of mathematician Emmy Noether in the early 20th century allowed us to make one of the most fundamental connections in our understanding of the universe: the relationship between symmetries and conservation laws.
Breaking space news, the latest updates on rocket launches, skywatching events and more!
Let's say you were to perform a physics experiment, haul your apparatus to a new location, repeat the experiment, and get the exact same result. Congratulations, you've identified a symmetry in space. And by Noether's Theorem, the law of conservation of momentum is obeyed in that situation.
For every such symmetry you can find, there is an associated conservation law. If you can rotate your experiment around and get the same results, you get conservation of angular momentum. Wait a few minutes and repeat? Conservation of energy. Hunts for these fundamental symmetries form the basis for … well, all of modern physics.
Empty space is perfectly symmetrical — moving objects inside it act as you would expect by obeying the usual momentum conservation laws. A normal crystal breaks spatial symmetry: There are spots on the crystal that look different than other spots (such as the edge of the object — otherwise there wouldn't be a crystal, would there?). And guess what: Conservation of momentum is not always obeyed inside a crystal. Two vibrations traveling in the same direction can intersect and combine, and the resulting wave can travel in the perfectly opposite direction.
This is called "umklapp" scattering, from the German for "flipped-over," and is now your official word of the day.
Taking time
Time crystals were first theorized in 2012 by Nobel laureate and certified smartie Frank Wilczek. After enough discussion and fleshing out by other theorists, the experimental physicists had enough to work with and began devising contraptions that would perform as a time crystal. Notably, it was roundly decided that such crystals could not exist in an equilibrium state, where all forces are perfectly balanced — after all, by definition, an equilibrium is characterized by an unchanging state, which means it would be perfectly symmetrical in time and couldn't possibly exhibit anything close to violating conservation of energy.
But nonequilibrium thermodynamics is a relatively new and hot field of physics, where systems are kept in a constant, perturbed state to see what happens, like constantly flicking your little sibling in the ear. And it's here that researchers were able to develop the first time crystal, by making an electron hop around a ring of molecules by zapping it with a laser. That may not sound impressive, until you realize that the electron hopped around at a particular frequency independent of the zappiness of the laser — an indication that the system had found its lowest-energy state, called a ground state, and that time crystalness had been achieved.
Since time crystals are not symmetric in time — they periodically cycle between states — conservation of energy is broken in their vicinity. That's right: The law of energy conservation, which says energy cannot be created or destroyed, and according to your high school physics teacher might as well have been the eleventh commandment, is violated by time crystals. [8 Modern Astronomy Mysteries Scientists Still Can't Explain]
Still right twice a day
Does this mean we need to rewrite our knowledge of the laws of physics? Well, the laws of physics, as interpreted by Wilczek and company, predicted the existence of time crystals, so I'm pretty sure we're on safe ground there.
[EMBED VIDEO: Video: Time Crystals are not Interesting at All]
Does this mean we have a perpetual-motion device? Well, kinda, and also kinda not. Since physical time crystals are not in equilibrium, they do require a driving mechanism to keep them happy. Turn off the laser, for example, and the time crystal falls asleep. Also, they are in their ground state, which means we can't pull any energy out of them.
What about conservation of energy? Well, that iron law always had an asterisk after its definition, the same as all the other conservation laws. In order for conservation to rule the land, the land must be symmetric, and symmetries can be broken in all sorts of ways.
Time crystals are a bit interesting, though, as far as symmetry-breaking goes. Before their construction, all other symmetries of nature had been found to be broken somehow, somewhere, except for the one connected to conservation of energy. But now we have an example of that symmetry being broken, too. Which is neat.
Learn more by listening to the episode "What's the deal with time crystals?" on the Ask A Spaceman podcast, available on iTunes and on the Web at http://www.askaspaceman.com. Thanks to Kiet N and Ben G. for the questions that led to this piece! Ask your own question on Twitter using #AskASpaceman or by following Paul @PaulMattSutter and facebook.com/PaulMattSutter.
Follow us @Spacedotcom, Facebook and Google+. Original article on Space.com.

Paul M. Sutter is a cosmologist at Johns Hopkins University, host of Ask a Spaceman, and author of How to Die in Space.