What is nuclear fission?
Not only does nuclear fission provide the majority of the electricity that powers our homes, but it has also proved how destructive the power within the atom can be.
Nuclear fission is the process of breaking large atomic nuclei into smaller atomic nuclei to release a large amount of energy.
This process is usually done by forcing the nuclei to absorb neutrons — the particle usually found in the atomic nucleus with protons. The phenomenon has been harnessed by humanity to both provide energy via nuclear power plants, but also to power nuclear weapons.
Fission is a form of nuclear transmutation, meaning that the starting atoms are not the same elements as the resultant — or daughter — product atoms. The fission process can occur spontaneously as a type of radioactive decay but this is rare, incredibly slow, and restricted to very heavy chemical elements.
Related: What is nuclear fusion?

Robert Lea holds a bachelor of science degree in physics and astronomy from the U.K.'s Open University. Robert has contributed to Space.com for over a decade, and his work has appeared in Physics World, New Scientist, Astronomy Magazine, All About Space and more.
Nuclear fission is the process of splitting atomic nuclei into smaller nuclei, releasing large amounts of energy as a result. Nuclear fission can help humankind meet its energy needs when chain reactions are controlled in reactors. Nuclear power now provides an estimated 85 percent of the electricity we use.
When this process is allowed to run unchecked, however, it gives rise to a powerful and destructive force. The detonation of so-called 'atom bombs' is signified by the sight of a mushroom cloud — a dreadful reminder of the power of the atom and of fission itself.
When was nuclear fission discovered?
The discovery of induced fission wouldn't have been possible without the strides made by Ernest Rutherford and Niels Bohr toward a coherent picture of the atom during the 1910s.
This led to the discovery by Henri Becquerel, Marie Curie, Pierre Curie, and Rutherford that the atoms of elements could 'decay' and transmute to another element via the emission of an alpha particle.
Two years after the discovery of the neutron in 1932 by James Chadwick, Enrico Fermi and his colleagues in Rome began pelting these newly found particles at uranium with other physicists also reaching the conclusion the particle would make a good probe of the atomic nucleus.
In 1933, Hungarian physicist Leó Szilárd first formalized the idea that neutron-driven fission of heavy atoms could be used to create a nuclear chain reaction having generated energy by using protons to split lithium the year before.
Finally, in December 1938, physicists Lise Meitner and Otto Frisch realized that isotopes of barium that appeared mysteriously during neutron-uranium bombarding experiments conducted by colleague Otto Hann were the result of the uranium nuclei undergoing fission.
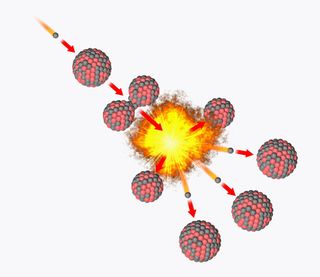
How does nuclear fission produce energy?
Induced nuclear fission occurs when a particle — commonly a neutron — passes a large target atomic nucleus and is captured by it. In nuclear reactors, this is an isotope — an atom with a different neutron count in its nucleus — of the heavy elements uranium or plutonium.
The energy needed to kick start fission is around 7 to 8 million electronvolts (MeV), and when a neutron carrying this level of energy or more strikes the target nucleus, the energy it imparts deforms the nucleus into a double-lobed peanut-like shape.
The gap between the lobes created by neutron capture eventually exceeds the point at which the strong nuclear force — which binds protons and neutrons together in the atomic nucleus and is powerful only across tremendously small ranges — can hold them together.
As a result, the nucleus fractures into smaller fragments , usually around half the mass of the starting particle, also releasing at least two, sometimes three, neutrons.
The daughter particles are rapidly pushed apart as a result of their positive charges repelling one another. The released neutrons traveling at a speed of around 33 million feet per second (10 million meters per second, or about three percent of the speed of light) — go on to strike two more nuclei, causing them to split and release four neutrons. Those neutrons are then ejected, striking other nuclei.
This leads to a chain reaction of splitting nuclei, producing a doubling of fission reactions each time a nucleus is split. That means by the tenth 'generation,' there are 1,024 fissions, and by generation 80 there are 6 x 10²³ fission reactions.
The reason this process releases energy is related to Albert Einstein's discovery that mass and energy are interchangeable. In its simplest form, this is encapsulated by arguably the world's most famous equation: energy equals mass times the speed of light squared or e=mc².
When a fissile material absorbs a neutron and breaks apart, the mass going into the reaction is slightly higher than the mass than emerges from it. The difference in mass between the starting particle and its daughter particles is tiny — about 0.1 percent of the original mass.
This is when the term c² becomes important as this tells us that even a tiny amount of mass liberates a lot of energy.
Around 85 percent of this energy liberated in fission reactions is released as kinetic energy granted to the daughter nuclei. This energy is then converted to heat. The rest of the energy is transferred as kinetic energy to the released neutrons or carried away by high-energy radiation in the form of gamma rays.
The precise daughter products created in fission can't be accurately predicted, as the process is subject to a high degree of chance and variation. In fact, so much so that there's no firm guarantee the capture of a neutron will happen or that this will even lead to fission.
One certain thing is that the number of protons and neutrons that goes into the process will be preserved at its conclusion.
One common reaction in nuclear reactors is the capture of a neutron by uranium-235 which creates two daughter neutrons and atomic nuclei of barium-144 and krypton-90. This reaction releases about 200 megaelectronvolts (MeV) which is equivalent to just 0.000000000032 Joules.
It's those created neutrons that are responsible for making fission a viable energy-generating mechanism. But this has to be strictly controlled.
Chain reactions and critical mass
Not all of the neutrons created in fission are available to drive further reactions, as some can be lost as fission proceeds. If enough neutrons can be maintained, however, the fission reaction becomes self-sustaining with this point described as 'critical mass.'
This self-sustaining critical mass point in nuclear fission is determined by several factors within the fissile material itself including its composition, its density, how pure it is, and even the physical shape it is arranged in.
Spheres have been found to minimize neutron loss that can prevent critical mass from being reached, which can also be reduced by surrounding the fissile material with a 'neutron reflector' which bounces back any stray neutrons.
One of the key aspects of making fission safe is controlling the chain reaction and the rate of fission. If less than one neutron from a fission reaction causes a further reaction, this can lead to fission running out of control and an explosion.
That means limiting the number of neutrons available to go on to create further fission reactions. In many reactors, this is done by introducing material that can 'soak up' neutrons, allowing the chain reaction to be sustained while also preventing fission from running out of control.
'Control rods' composed of boron or cadmium — elements that are strong neutron absorbers — or a mix of both are a common mechanism for controlling power levels in fission reactors. Power can be increased by slightly withdrawing control rods and allowing neutrons to drive up reactions. when the desired power level is reached, control rods can be re-inserted to stabilize reactions.
In some reactors, water infused with boron is used as a coolant with its concentration reduced as fission created neutron absorbing by-products.
Related: US military wants to demonstrate new nuclear power systems in space by 2027
Water can also be used to strip the energy away from fast neutrons released with too much kinetic energy. This makes these neutrons more likely to go on to trigger fission or to be absorbed by control rods.
Delayed neutrons — created at any time after fission ranging from a few milliseconds to minutes — are also important in preventing chain reactions from running out of control.
Produced in small amounts, delayed neutrons have less energy than immediately emitted 'prompt neutrons,' and without them the fission chain reaction would be unbalanced, leading to a virtually instantaneous and uncontrollable rise or fall in the neutron population.
Atom bombs are powered by a mass of fission nuclei assembled instantaneously and held together for about a millionth of a second. This allows the chain reaction to rapidly spread through the fissile material showing what happens when chain reactions are not controlled.

Is nuclear fission safe?
After the world witnessed the detonation of atomic bombs and the destruction and loss of life that they wrought in the bombings of Hiroshima and Nagasaki in August 1945, it is little wonder that the general public is wary of nuclear power.
Despite prominent and famous examples of nuclear fission accidents throughout history such as those at Three Mile Island, Chernobyl, and Fukushima, this source of energy is safer than ever.
In 2022, Our World in Data reported that for every terawatt-hour of energy generated by fission there are just 0.07 deaths, compared to 32.7 deaths for the same amount of energy generated by fossil fuels.
Even those infamous accidents themselves may have claimed fewer lives than their terrible stain on history would have most of us believe.
The World Nuclear Association says that the 2011 Fukushima accident, caused when a magnitude-9 earthquake triggered a 50-foot (15-meter) tsunami that disabled the plant's power supply and cooling mechanisms, claimed zero lives as the result of radioactive material leaks.
Likewise, according to the World Nuclear Association, the 1979 Three Mile Island accident in Pennsylvania caused no deaths as a result of the leak of radioactive gas caused by a cooling malfunction.
Arguably the world's most famous nuclear accident occurred at the Chernobyl Nuclear Power Plant, near the city of Pripyat in Ukraine in 1986 as a result of a flawed reactor design that was operated with inadequately trained personnel.
This resulted in two workers being killed in an explosion and a further 28 people dying within weeks of the accident. The World Nuclear Association also attributes over 5,000 thyroid cancer cases, including 15 fatalities, to the accident. To this day, a 1,000-square-mile (2,600-square-kilometer) uninhabited exclusion zone remains around the former plant.

One of the reasons for the impressive safety of current fission power plants is that high-profile accidents like those listed above have prompted the development of improved designs and safety features.
The current iteration of fission plants are Generation III reactors. These are notable for several features, particularly a reduced possibility of core-melt accidents.
Many safety features are inherent to the designs of these reactors, for example, fast neutron reactors operate using a system that slows as temperature increases.

Is nuclear waste dangerous?
One common myth about nuclear power is that 'nuclear waste,' the radioactive by-products of fission processes, lasts forever.
While there is no doubt that the safe storage and disposal of fission by-products is a concern, much of this material is actually recyclable and has been responsibly managed since the onset of civil nuclear power.
The World Nuclear Association (WNA) says fission reactors create a small amount of waste that comes in three types, ranked based on their level of radioactivity from low, to intermediate, to high-level.
The organization adds that 90 percent of fission waste fits in the first low radioactivity category. High-level nuclear waste accounts for 3 percent of total waste but releases 95 percent of the radioactivity of fissile waste.
Despite the picture of hazardous nuclear waste popularized by "The Simpsons" and other pop-culture staples, this waste isn't a glowing green ooze. Rather most of this is 'spent fuel' in the form of metal rods containing ceramic pellets of enriched uranium.
Spent nuclear fuel can be recycled to create new fuel and byproducts, with the Office of Nuclear Energy suggesting that it retains 90 percent of its potential energy even half a decade after use in a reactor.
Currently, while countries like France recycle spent nuclear fuel, the United States doesn't do this, though plans are underway for reactors that could operate with spent fuel.
In the United States, used fuel rods are enclosed in steel-lined concrete pools of water or are encased in steel and concrete containers and then stored at 76 different reactor sites across 34 states. This spent fuel waits here for a permanent disposal solution.
Additional Reading
Humanity shouldn't ever forget the potential for destruction presented by nuclear fission. Father John A. Siemes, professor of modern philosophy at Tokyo's Catholic University, gives an eyewitness account of the detonation of an atom bomb over Hiroshima.
The last boson of the standard model to be discovered, the Higgs boson, determines how other particles get their mass.
Bibliography
"Nuclear Fission: Basics." Atomic Archive (2022).
"Nuclear fission." Britannica (2022).
"Fissile Elements: Supply and Demand." MIT (2022).
"Physics of Uranium and Nuclear Energy." World Nuclear Association (2022).
"Physics and Kinetics of TRIGA Reactors." IAEA (2022).
"Fukushima Daiichi Accident." World Nuclear Association (2022).
"Three Mile Island Accident." World Nuclear Association (2022).
"Chernobyl Accident 1986." World Nuclear Association (2022).
"What is nuclear waste, and what do we do with it? World Nuclear Association (2022).
"5 Fast Facts about Spent Nuclear Fuel" Office of Nuclear Energy (2022).
"Nuclear Energy." Our World in Data (2022).
"This Month in Physics History." APS Physics (2007).
Follow us on Twitter @Spacedotcom or on Facebook.
Join our Space Forums to keep talking space on the latest missions, night sky and more! And if you have a news tip, correction or comment, let us know at: community@space.com.
Get the Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!

Robert Lea is a science journalist in the U.K. whose articles have been published in Physics World, New Scientist, Astronomy Magazine, All About Space, Newsweek and ZME Science. He also writes about science communication for Elsevier and the European Journal of Physics. Rob holds a bachelor of science degree in physics and astronomy from the U.K.’s Open University. Follow him on Twitter @sciencef1rst.
