Interstellar Ice Acts Like a Liquid in Ultraviolet Light
When exposed to ultraviolet light, interstellar ice may act more like a liquid than a solid, a new study has found.
Researchers discovered this effect while re-creating the conditions of our early solar system's planet-forming disk in a laboratory environment, revealing how organic chemistry might react to the deep freeze of the system's outer regions and how the seeds of planets accumulate material.
It is well known that water ice and organic chemistry are found in comets and asteroids. Comets coalesced from the primordial materials found in our solar system's pre-planetary disk of dust and gas, called a protoplanetary disk, around 4.6 billion years ago, and asteroids contain interesting chemistry that is currently the focus of NASA's OSIRIS-REx asteroid-sampling mission. [Photos: Spectacular Comet Views from Earth and Space]
As these materials date back to the solar system's early days, they can be studied to understand how the planets accreted material and how prebiotic chemistry formed in the solar system's ancient protoplanetary environment.
To better understand the characteristics of interstellar ices, new work — led by researchers at Hokkaido University in Sapporo, Japan — studied mixtures of water (H2O), methanol (CH3OH) and ammonia (NH3) gases at very low temperatures and exposed them to ultraviolet (UV) radiation. Young stars generate powerful UV radiation, so any chemistry in the protoplanetary disk surrounding a young star (or protostar) would likely be affected by this radiation, the researchers hypothesize.
Inside a low-temperature photolysis apparatus called PICACHU (Photochemistry in Interstellar Cloud for Astro-Chronicle) at Hokkaido University, these gases condensed at low temperatures and formed layers of ice on a gold-coated copper substrate. Then, they exposed the ice to UV radiation while the temperature of the apparatus was raised. After the ice was irradiated, the researchers observed strange features in the residue.
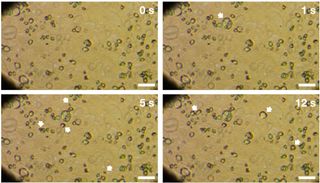
"We first saw big holes on our organic residue — which formed from the UV irradiated ice — and wondered how they formed," lead study author Shogo Tachibana, of Hokkaido University, told Space.com.
Get the Space.com Newsletter
Breaking space news, the latest updates on rocket launches, skywatching events and more!
After seeing the holes left in the samples, Tachibana and his team decided to observe the ices in situ during the experiments, and saw the ice bubbling "like a liquid" in videos, he said.
"It was so surprising to see such a liquid-like behavior," Tachibana said.
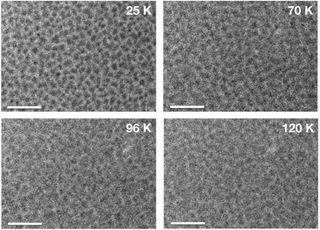
The organic samples bubbled at between 65 and 150 Kelvin, whereas the pure water ice samples bubbled at between 50 and 140 Kelvin. Fewer bubbles appeared in samples containing more water ice. The bubbling behavior was not observed in ice that was not irradiated by UV.
This liquid-like behavior has not been seen before, but the researchers think the liquefication occurs at low temperatures because the UV photons break the atomic bonds of the molecules in the ice, Tachibana said. This so-called "radical formation" may increase the microscopic mobility of the chemicals, creating a viscous or liquid-like state, he said.
This finding has implications for the ices found in protoplanetary disks, Tachibana added. Astronomical observations have detected water, methanol and ammonia around young stars, so an interstellar "analogue" created in the lab could reveal a process that occurs on tiny scales when these ices are exposed to powerful UV radiation from protostars.
Because liquid is a good reaction medium, Tachibana said, the formation of organic molecules may be enhanced in the liquid-like ice, because they will have much higher mobility than molecules in a solid.
"If this is the case, the formation of organic molecules, including prebiotic ones, may start at the cold environment of the disk around 50 to 60 Kelvin, which is far beyond the snow line," he added.
The snow line (or frost line) is the distance from a protostar where the temperature is low enough for volatiles (like water, carbon dioxide, ammonia, methane and methanol) to condense and freeze into solid grains of ice. Beyond this distance, nothing should be in a liquid state. But as the protostar pumps out UV light, according to this research, it might be possible for these ices to exhibit liquid-like properties. This, in turn, might create a surprisingly reactive environment for organic chemistry to thrive, perhaps explaining the abundance of organics found in comets.
It could also provide an important clue as to how planets formed. Current theories suggest planets form when small particles in protoplanetary disks surrounding young stars collide and clump together. Eventually, this clumping forms larger and larger objects that possess stronger and stronger gravitational fields. But the genesis of this process is poorly understood. If the ice grains in cold regions of protoplanetary disks have liquid-like qualities, this could result in the rapid formation of planets, the researchers wrote.
The new work was detailed Sept. 29 in the journal Science Advances.
Follow Ian O'Neill @astroengine. Follow us @Spacedotcom, Facebook and Google+. Original article on Space.com.
Join our Space Forums to keep talking space on the latest missions, night sky and more! And if you have a news tip, correction or comment, let us know at: community@space.com.
Ian O'Neill is a media relations specialist at NASA's Jet Propulsion Laboratory (JPL) in Southern California. Prior to joining JPL, he served as editor for the Astronomical Society of the Pacific‘s Mercury magazine and Mercury Online and contributed articles to a number of other publications, including Space.com, Space.com, Live Science, HISTORY.com, Scientific American. Ian holds a Ph.D in solar physics and a master's degree in planetary and space physics.
